Intellia Therapeutics is about to unveil phase II data on NTLA-2002 at the end of the month. NTLA-2002 aims to be a one-time treatment for hereditary angioedema (HAE). Prior phase I data looked intriguing with all 10 subjects becoming essentially attack free in the months following (1-time) drug administration and safety findings limited to around the time of treatment.
Still, the
study involved too few patients for too short time to clearly define the
commercial product in terms of dose, safety, and efficacy (including durability).
HAE is a
rare disease with an estimated incidence of 1:10000 to 1:50000 depending on geography.
These angioedema attacks are characterized by tissue swelling due to the influx
of liquid into tissues through leaky vasculature as a result of bradykinin
generation. With few exceptions, they occur in people due to their genetic lack of C1 esterase inhibitor
function. HAE
must be differentiated from allergic edema and as such is not responsive to
antihistamines or corticosteroids.
In addition
to outwardly visible soft tissue swelling, e.g. of the lips and hands, it can also
involve internal systems like the abdomen and lead to excruciating pain. Suffocation from laryngeal edema is probably the most feared disease manifestation. It
is therefore not surprising that many patients become depressed, stop engaging
in normal daily activities to avoid anything that may set off attacks,
including stressful situations, physical activity and injury (including
surgery).
Rapid progress in the understanding of the biology behind HAE has resulted in a number of treatments for on-demand and prophylactic uses and significantly improved the outlook for patients. Still, there is widespread dissatisfaction with the incomplete protection and requirement for regular drug
administration of current prophylactic treatments while the
utility, convenience, side effects and even embarrassment that come from taking
on-demand drugs is being questioned.
Switching between drugs and treatment approach is common and with costs often on the order of
$500,000 per annum, access can be a real issue causing additional anxiety.
Enter
NTLA-2002
NTLA-2002
promises to address all these challenges.
That’s a
bold statement, but the phase I clinical data provide hints that
this could become reality, commercially in 2027 if you are living in the US or
even earlier if you decided to and qualified for the global pivotal clinical trial
that has just commenced.
NTLA-2002
is a CRISPR-based genome editing treatment that permanently and selectively disrupts the KLB1
gene in liver hepatocytes. KLB1 codes for plasma prekallikrein (PKK) which is a critical component in the
kallikrein-kinin inflammatory system.
Importantly, there are people that naturally lack plasma PKK (Fletcher’s
syndrome) or another key component, Factor XII, and these appear to be
perfectly healthy. They are typically only identified by coincidence following
routine lab tube tests showing prolonged activated partial thromboplastin time without an actual negative impact on bleeding or otherwise in the natural context.
NTLA-2002
comprises of a messenger and guide RNAs entrapped in liposomal nanoparticles
(LNPs) similar to the covid mRNA vaccines. Following one-time intravenous
administration, these traffic to the liver hepatocytes where the guide
RNA directs the CRISPR Cas9 enzyme to disrupt the KLB1 gene.
Such a
formulation already exists commercially in the form of ONPATTRO. ONPATTRO is an RNAi
therapy that is administered every 3 weeks. Similar to the phase 1 study of NTLA-2002 (Longhurst et al 2024), transient immune suppression
(corticosteroids, antihistamines) is practiced around the i.v.
administration. The great thing about
NTLA-2002 is that the side effects from this procedure are well recognized and
managed and would only present once- if at all.
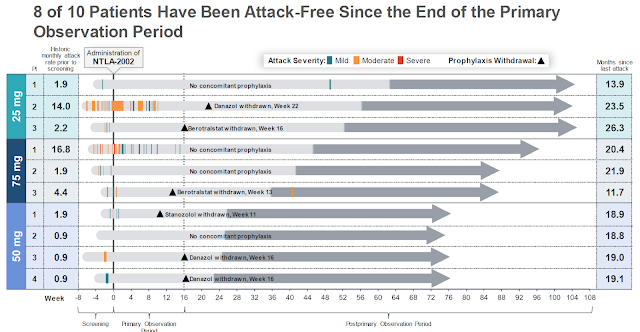
As mentioned above, all10 subjects in the dose-ranging phase I study of NTLA-2002 have essentially become attack free once the medicine had a chance to do its work, destroy hepatocyte KLB1 and normalize the kallikrein-kinin-system. This indicates that after the optimal dose has been found, a good number of HAE patients may go on with life and forget about their disease! So patients may not only enjoy the best efficacy of any HAE treatment, they would only have to take it once.
The phase II study results will more than triple the number of patients that have been (hopefully successfully) exposed to NTLA-2002 and thus provide a good basis for the dose chosen to undergo confirmatory phase 3 testing. Based on recent statements, the company will select the 50mg dose which in the phase I study gave -88% plasma PKK reduction, a level well above the -45% reduction where HAE attack rates start to drop off precipitously (Riedel et al 2024).
For those
that continue to experience a rare attack, it may be possible to give
another dose of NTLA-2002. Data from another
(monthly administered) antisense oligonucleotide therapeutic (donidalorsen)
targeting the same PKK gene indicate that attack rate reduction and PKK
inhibition are tightly correlated (Riedel et al 2024). It is therefore conceivable that once plasma PKK
drops below a critical concentration in the blood, swelling attacks are virtually
eliminated. A presentation at the upcoming ACAAI
meeting by the sponsor of donidalorsen, Ionis Pharmaceuticals, should further shed light on this.
Medical
innovation is costly and NTLA-2002 which promises to be the second approved CRISPR
genome editing therapeutic following Casgevy for sickle cell disease will be no different. Given the rarity of HAE, it would not be
surprising if it cost 2 to 3 million USD for treating a patient. This compares to other medications in the
space, including Cinryze or Takzhyro, which often carry annual price tags exceeding
$500,000. Sure, healthcare
administrators are likely to throw up their hands and have sticker shock, but
simple math shows a one-time treatment to be a real money saver for patients
that have decades of disease-free lives in front of them.
Disclosure: this is not medical advice. Always
talk to your HAE specialist doctor first before making a treatment decision.