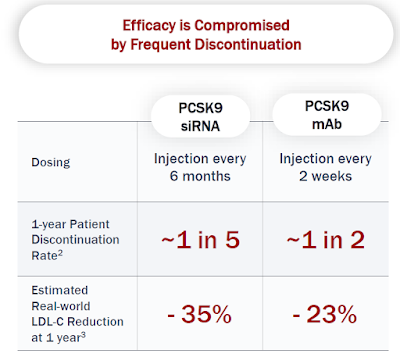
Patients do not benefit from drugs they do not take. This is especially true in the cardiovascular disease space aimed at lowering atherogenic LDL-cholesterol where the majority of patients starting on oral options like statins do not take their pills after just one year. This is also true for once every 2 to 4 weeks next-generation PCSK9 antibodies and even semiannual PCSK9 RNAi therapeutic inclisiran, though to a lesser degree in the latter case.
With this realization in mind, Verve Therapeutics set out to develop a PCSK9 CRISPR base editing treatment that should lower LDL-cholesterol for life by at least -40% after just a single administration of an intravenous LNP formulation. Unfortunately, a first generation formulation, VERVE-101, had to be abandoned a year ago because of laboratory abnormalities, in particular ALT/AST elevations 5 to 10-fold above the upper limit of normal as well as a case of dangerously low platelet counts in a first clinical trial. In addition, the intra-dose variability of the PCSK9 knockdown and LDLc lowering between subjects and the dose-responsiveness were not optimal.
All evidence pointed towards the LNP formulation, not the PCSK9 as the target or the base editing process, to be the culprit for the safety issues. Verve therefore decided to replace some of the lipids in the liposomal formulation and add GalNAc sugars so as to allow the LNP to be taken up by both the LDL-receptor (via ApoE)- and ASGPR (via GalNAc). This is helpful for two patient populations that are most in need for new treatment options and which lack LDL receptors (heFH and hoFH). The base editor and guide RNAs were left unchanged from VERVE-101.
Based on data from the first 14 subjects treated with VERVE-102 revealed today the theory translated perfectly into clinical practice. At doses above 50mg of the LNP, the mean LDLc reduction was -59%, in line with the most potent PCSK9 agents (antibodies) and significantly more potent than inclisiran, especially in the heterozygous FH (heFH) population. Moreover, there was a beautiful dose response for both PCSK9 and LDLc lowering and very little inter-patient variability.
Dose-related LDLc lowering in the HEART-2 trial with VERVE-102
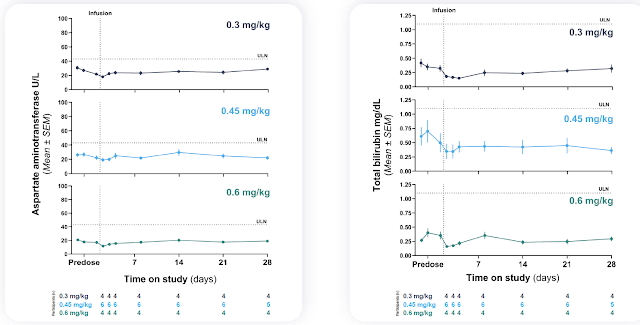
Even more importantly, the safety was pristine. There was hardly a blip with no outliers in terms of ALT/AST changes upon LNP administration, a stark difference to VERVE-101. Similarly, no platelet changes were seen. Only a single case of grade 2 infusion reaction was observed which rapidly resolved and does not pose an obstacle to further clinical development and commercialization. Anybody familiar with LNP technology understands that GalNAc-LNPs are now the gold standard in the delivery of genome editing in the liver.
Verve Therapeutics is wrapping up the HEART-2 study with a final higher dose to see whether there is further LDLc lowering and then proceed to a ~60-subject phase II study aimed at locking down one of two fixed doses of VERVE-102 for the registrational phase of clinical development.
Today marks a milestone in moving genome editing to large, indeed very large patient populations.
Disclosure: I owned some Verve Therapeutics shares going into data and doubled down on it after seeing the emerging VERVE-102 product profile.



4 comments:
It all sounds good, but we still don't have any evidence that the effect won't fade after a couple of years to we? I started using the Inclisiran in June of 2022 and it took my LDL from about 165 to 65 and has pretty much kept it there for almost 3 years now. Twice a year isn't too bad and I have no side effects. I think the wholesale price on Leqvio is $3,250 per injection, but they charge me about $4,375 after the markup, so almost $9,000 per year. Have they discussed what they might charge for the one time treatment? Thanks for your comments and good luck on the investment. Verve would certainly be a better play than NVS.
It all sounds good, but we still don't have any evidence that the effect won't fade after a couple of years to we? I started using the Inclisiran in June of 2022 and it took my LDL from about 165 to 65 and has pretty much kept it there for almost 3 years now. Twice a year isn't too bad and I have no side effects. I think the wholesale price on Leqvio is $3,250 per injection, but they charge me about $4,375 after the markup, so almost $9,000 per year. Have they discussed what they might charge for the one time treatment? Thanks for your comments and good luck on the investment. Verve would certainly be a better play than NVS.
Thanks, Steve, for your comments. How do you feel about cost and reimbursement of your current therapy and having to turn up twice (?) in the docs office annually for the injection? Would a one-time treatment be attractive to you? I have not started on LDLc-lowering medication, but with a chronically elevated LDLc in light of a close to 40% risk of dying from CVD in Western societies, I definitely should in the next 5 years. A one-time-then-forget-about-it therapy is definitely attractive to me and personally would be willing to foot something on the order of $100k.
I actually go to a place that usually does infusions, but they make it pretty easy since I have had several doses with no issues. Takes less that 10 minutes. I have been able to get insurance to pay for most of it, but I have to put it on my credit card first and then file with the insurance company and wait a couple months before they pay me back. At my age, 76, it probably makes sense to just do the 2x per year cost wise. Also, are we sure that the "one time" therapy will really be a one and done? I wonder if I could have avoided some of my heart issues if either one of these had been available 20 or 30 years ago. Oh well, too late for that on my end anyhow. At age 50 or younger, the one and done would have real appeal.
Post a Comment