Alpha-1-antitrypsin disease (AATD) is caused by mutations in the SERPINA1 gene coding for alpha-1-antitrypsin (AAT). There are an estimated 100000 alpha-1 patients in the US alone, making it a rare, but not ultra-rare disease.
Correcting these mutations, replacing AAT through gene
therapy, or inhibiting the particularly pathogenic Z-allele is subject to the
efforts of a number of nucleic acid-based drug developers, most notably Arrowhead
Pharmaceuticals (RNAi), Wave Life Sciences and Korro Bio (RNA
Editing) as well as CRISPR genome editing companies Beam Therapeutics
(Base Editing) and Intellia Therapeutics (CRISPR Cas9 and targeted gene
insertion).
The protein name is somewhat misleading as it’s main function is to antagonize neutrophil elastase activity in the lung. Insufficient AAT activity can lead to lung injury during pulmonary stress, especially respiratory infections or smoking. Historically, lung disease has been addressed by smoking cessation and preventing lung infection (e.g. through vaccination).
In those patients that do progress to symptomatic lung
disease, the standard of care still remains weekly infusions with
plasma-derived (!) alpha-1-antitrypsin.
Although the evidence of benefit is substandard, partly the result of having a ‘good-enough’
therapy approved decades ago on simple biochemical measures, this
is now a $1B+ market and growing with the increased identification of this
genetic form of chronic obstructive pulmonary disease (COPD). Growing awareness of the liver aspect of the genetic condition also contributes to better patient identification.
Achieving 50-60% of normal AAT activity is expected to
be therapeutic based on human genetics.
As lung health and longevity has been improving in
alpha-1, it is estimated that 15% of adult patients develop the serious
condition of liver cirrhosis. There is also an infant form AAT liver disease manifesting in ~10% with alpha-1 mutations that can result in cirrhosis and the need for liver transplantation. Although this is less well understood than the adult form, it is likely to involve excessive accumulation of alpha-1-antitrypsin in the liver. These patients typically carry the Z allele on both chromosomes (piZZ). This allele is particularly prevalent among the European and US AATD populations
(~90% of 235k worldwide). As true AAT
null mutations are rare, piZZ is by far the most prevalent genotype of patients
presenting with either lung or liver disease.
The Z alpha-1 antitrypsin variant protein misfolds,
aggregates and gets stuck in the endoplasmic reticulum of the liver. This results in
liver inflammation and progressive injury (cirrhosis, hepatocellular carcinoma/HCC). A small fraction (10-15%) of Z-AAT does get
exported, but even if it makes it to the lung it is less functional and may even
exacerbate lung inflammation due to its propensity to precipitate.
Removing Z-AAT is expected to halt and even reverse
liver disease based on human genetics (1 Z allele not sufficient to cause
disease) and preclinical animal models.
Here I will discuss some of the more promising, new innovative approaches utilizing a number of nucleic acid therapeutics modalities to address the lung and/or liver complications of AATD. It also explains the following rank order of the approaches in terms of promise for AAT lung and liver disease.
1. RNAi for A1AT-related liver
disease (Arrowhead Pharmaceuticals and Takeda)
While innovation in the lung space of AATD has stalled, the disease attracted fresh attention in the pharmaceutical industry about a decade ago for its
liver-related complications as (piZZ) alpha-1 patients get older and increasingly
suffer from liver failure and HCC. This time also saw the rise of new therapeutic modalities such as RNAi.
The RNAi Therapeutic ARO-AAT by Arrowhead Pharmaceuticals has demonstrated almost complete elimination of the highly expressed gene in phase I
and II clinical trials. In preclinical
models, this has been shown to reduce existing liver Z-AAT aggregates and
inflammation over time.
In a small (n=25), 2:1 ARO-AAT/placebo randomized phase
II study in Z-AAT patients with liver fibrosis, there was a robust -68%
reduction in liver AAT globule burden. At 52
weeks, this translated to 50% of subjects on ARO-AAT having a 1 or more point improvement
in Metavir fibrosis (scale: 0/no fibrosis to 4/cirrhosis). Due to the small study size and the 3/8
responses (38%) in the placebo group, this did not reach statistical significance. Another small open-label study demonstrated a
similar 50% fibrosis response.
Importantly, RNAi knockdown of Z-AAT did not worsen pulmonary health over 1 year in the controlled (no smoking etc) trial setting. This may be partly due to Z-AAT being functionally impaired anyway and may do more harm than good in the lung.
A longer 2 year 160 patient pivotal phase 3 study with F2-4
disease is underway to statistically confirm the benefit of
ARO-AAT (aka TAK-999) on liver fibrosis (in F2+3 patients). Results from that study can be expected in
early 2026.
2 years should be plenty of time for liver globules to
turn over. And as the liver is good in
regenerating once you take the fibrogenic trigger away (see HCV, NASH etc)- as long as the liver is not in a late-stage cirrhotic stage already- this should translate into a fibrosis benefit.
For AAT liver disease, Arrowhead enjoys at least a 5 year headstart over the non-RNAi competition which has yet to enter the clinic. Dicerna, now a Novo Nordisk company, has also been developing an RNAi trigger (belcesiran) for AAT which is in a phase II and has demonstrated -77% knockdown following a single dose.
In terms of efficacy, Arrowhead’s RNAi approach with it’s almost complete elimination of AAT in the liver appears to be substantially superior over the competition with non-RNAi approaches struggling to get to -70-75% Z-AAT reductions. This assumes that more knockdown is correlated with efficacy or at least the time for efficacy to manifest. Based on human genetics, the equivalent of life-long treatment, where subjects with just one Z allele have not much added risk of developing liver disease, this may not be necessary.
2. CRISPR Cas9 for Liver Disease
The non-RNAi approach in development for AAT liver disease that is likely the next most efficacious one is CRISPR Cas9 DNA cleavage as developed by Intellia Therapeutics. Intellia has already demonstrated 90%-type gene knockout efficiencies in hepatocytes for TTR amyloidosis and hereditary angioedema in the clinic, so expectations are for similar target engagement in the AAT program. Cas9-targeted DNA cleavage is certainly a powerful tool to downregulate gene expression.
The question, however, is whether you would want to
risk disabling a gene permanently when you have non-permanent alternatives like
RNAi that are at least equally efficacious.
Similar to transthyretin in TTR amyloidosis, alpha-1-antitrypsin is a highly
expressed gene in the liver that has important functions in human biology and
health. TTR for example is a carrier of thyroxin
and retinol binding protein (à
vitamin A) while AAT is involved in the homeostasis of protease activity for
example during infection in the lung and probably has similar homeostatic functions in other tissues.
Side effects from the long-term ablation of AAT may only
manifest after years and may then require life-long supplementation (vitamin A, thyroxin), or after certain stress situations (non-genetic COPD etc). To my
knowledge, unlike for say PCSK9, human genetics does not support that lifelong
absence of AAT is ideal. Similarly,
regulatory agencies will be worried about cancer resulting
from genome re-arrangements rearing their ugly heads 10 years or so down the
line following on- or off-target DNA cleavage in and outside liver cells
(including germline).
In my opinion, unless genome editing can demonstrate
clear efficacy advantages over otherwise quite safe and non-onerous, non-permanent alternatives, it is only with the accumulation of long-term safety data that we will be able to tell whether CRISPR Cas9 can be used more broadly.
3. RNA Editing for Lung and Liver Disease (Wave Life Sciences, Korro Bio)
When it comes to addressing AATD lung disease, my
favorite approach is via RNA Editing with an exciting candidate by Wave Life Sciences
(WVE-001) about to enter the clinic.
Korro Bio aims to enter a competing RNA Editing candidate into the
clinic in late 2024.
However, Korro Bio candidate would likely have to be relatively
frequently (weekly) administered via intravenous infusion and has no obvious
efficacy advantage over WVE-001 and LNP-related toxicities to be expected
(infusion reactions, triggering of innate immunity etc). As a second mover
with an inferior therapeutic profile, I am struggling to understand Korro Bio’s
business rationale here (also discussed here).
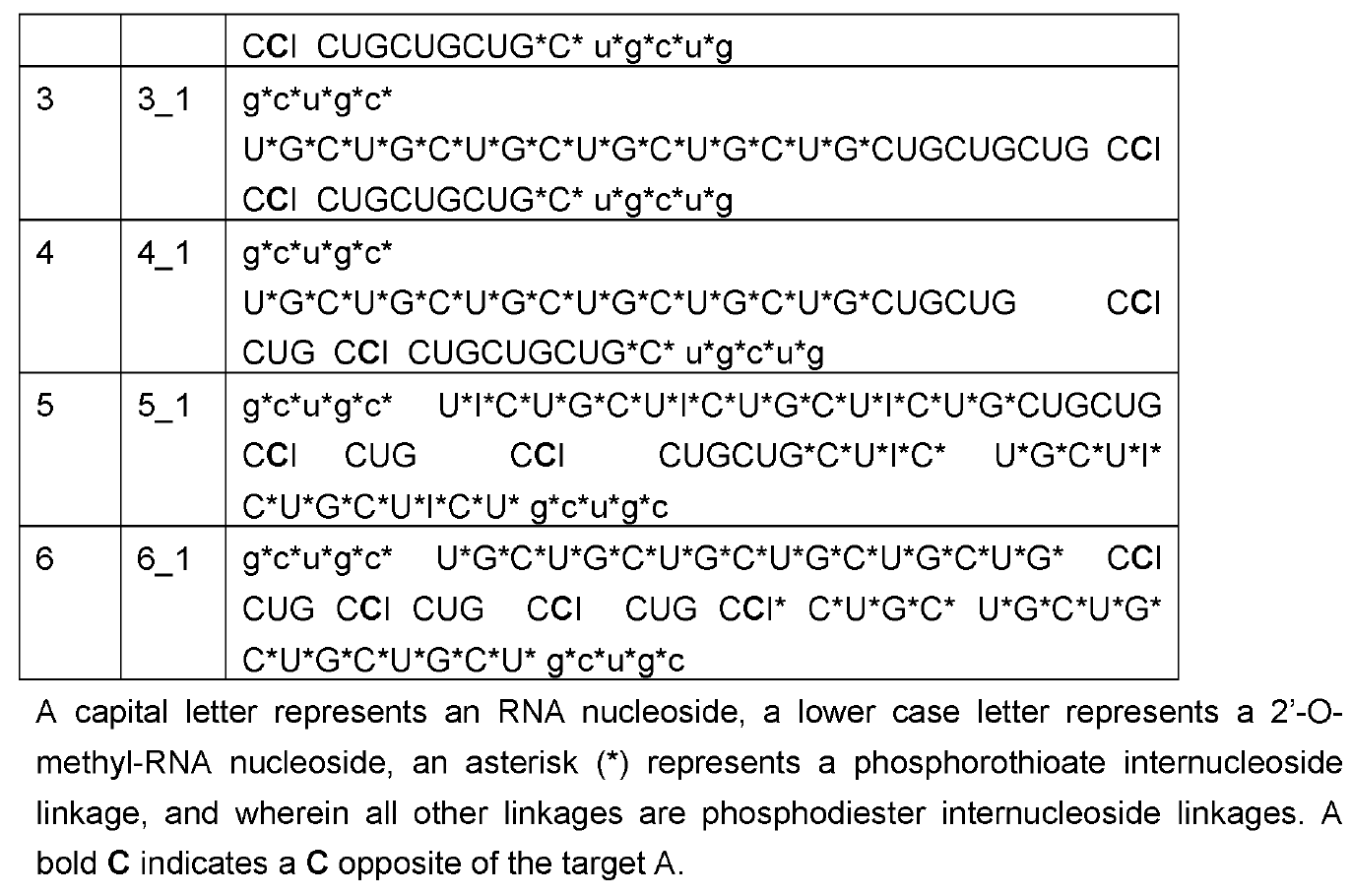
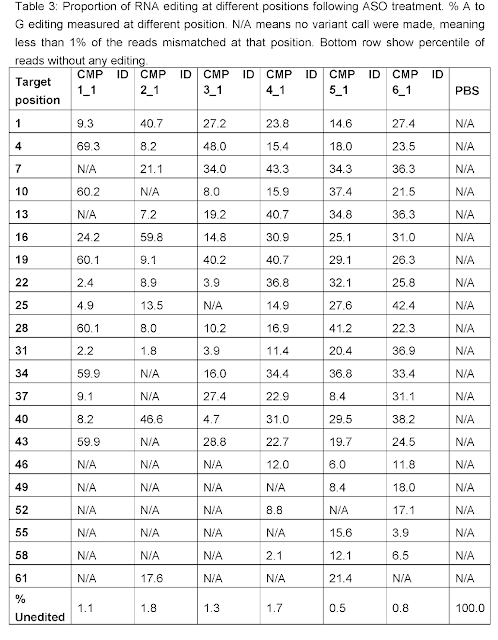
As discussed on this blog, RNA Editing in AATD aims to
convert the pathogenic piZ allele into the healthy M allele. Based on human genetics where MZ carriers are
protected from both liver and lung disease, a 50% AàI editing rate may suffice for both indications. Wave Life Sciences has been able to meet that bar in preclinical
studies. Given the newness of RNA
Editing, results from the first clinical study for this modality utilizing
Wave’s subcutaneously administered oligonucleotide chemistry will still have to show
just how efficacious and sustained (in terms of dosing frequency) their approach
will prove to be in humans.
A significant uncertainty is how long it will take for even robust >60% editing to translate into actual clinical benefit. Unlike for the liver where the natural
turnover of pathogenic globules will likely be rate-limiting for liver health
improvement to manifest, generating healthy, wild-type alpha-1-antitrypsin
should be immediately beneficial to the lung.
Identifying a patient population with still relatively healthy lungs but
rapid functional decline should therefore be most promising for a clinical
trial.
For this, Wave Life Science has
recruited pulmonary disease powerhouse GSK through a out-licensing as setting a
new standard of care will not be as easy as simply demonstrating AAT blood
biomarker as the incumbents had gotten away with. The fact that plasma-derived
products from the stone age of biotechnology still dominate what today has
grown into a blockbuster market shows how difficult it has been to find new
therapeutic strategies worth investing in. As the plasma-derived
products have been unable to demonstrate a clear therapeutic benefit in
controlled studies, this would likely involve running a relatively large and
long-term clinical study being very mindful of stratifying the diverse patient
population.
The reason why RNA Editing could represent a meaningful improvement to
the standard of care in AAT lung disease is that it promises to offer more
sustained, tonic amounts of AAT instead of the spiked pharmacokinetics from
weekly infused AAT. Moreover, because RNA Editing converts mutant to wild-type
AAT that is transcribed from the native gene under physiological controls (e.g.
promoter activity), adverse effects from supraphysiological levels of AAT
should not be observed.
Wave Life Sciences claims that RNA Editing can not only address lung AATD,
but also its liver manifestations. To find out, GSK would have to
run a separate study and hope that clinical success is not linear with Z-AAT
reduction. Because if this were the case, RNAi would likely win out over RNA
Editing not only in terms of knockdown efficacy, but also dosing
frequency. There is some preclinical evidence, however, that
wildtype AAT may aid cellular export of Z-AAT, so a 50% mRNA conversion may
reduce intracellular Z-AAT more than 50%. Possibly worth a competitive
gamble.
4.
Base Editing for Lung and Liver
Disease (Beam Therapeutics)
Similar to Wave Life Sciences, Beam Therapeutics aims to correct the
piZZ mutation, but this time applying CRISPR genome base editing for permanent correction. Unlike
traditional CRISPR Cas9, Beam’s CRISPR proteins are unable to make
double-strand. Instead, a base-editing enzyme is tethered to the Cas protein
which is guided to the target location by the guide RNA. In this
case, the base editor is an Adenine Base Editor converting proximal adenines
into guanine.
Unlike RNA Editing where you can precisely determine the editing site
through secondary fit and oligonucleotide modifications with essentially no
off-target editing, tethered base editors will act on what is close
by. Most problematic for the Beam program appears to be a bystander adenine
that is 2 bases away from the targeted adenine such that for each desired
single edit you seem to get equal amounts of double-edited AAT mRNAs resulting
in alpha-1 antitrypsin that is significantly less functional.
This also raises that question on how many more unidentified off-target
base edits there are. The genome in the nucleus is not linear, but
dynamic with tertiary interactions between chromosomes, within a chromosome and
transcriptional hubs bringing multiple genes into close vicinity. So
imagine a crowded nucleus with thousands of CRISPR-tethered base editors
floating around looking for adenine substrates. It will sure be
interesting how regulatory agencies around the world will be looking at this as
well as the risk of germline editing following LNP delivery.
Base editing efficiencies are slightly less than RNA Editing in
preclinical models with the added caveat around the double edits.
Beam intends to file for clinical trial applications in Q1
2024.
5.
Integrational Gene Therapy for Lung
Disease (Intellia Therapeutics)
Finally, in addition to gene demolition using Cas9 for liver disease,
Intellia is advancing a separate CRISPR-mediated gene insertional approach for
AAT lung disease. Here, an LNP delivers Cas9 mRNA-gRNA to open up
DNA downstream of the albumin promoter, highly active in
hepatocytes. An AAV carrying AAT cDNA gets administered alongside so
that a certain fraction gets incorporated as the DNA damage repair machinery
attempts to mend the lesion.
Preclinical non-human primate data support that marked expression can
thus be achieved as the albumin promoter now drives transcription of AAT-cDNA. It
is noteworthy that albumin drop-in has gone a bit out of fashion in the genome
editing field after Sangamo Biosciences had similarly claimed high preclinical
expression for hemophilia and lysosomal storage disease almost a decade ago.
Strangely, as so often has been the case with Sangamo, this could not be
reproduced at all in the clinic (barely detectable levels if
any at all). Intellia is using a curious inverted repeat design as
their drop-in cassette and believes this to be a game-changer in the
approach. They may have done this to double their chances that the
insertion happens in the correct orientation, but as a former molecular
biologist trained in RNA polymerase II transcription and RNAi this looks like
inviting trouble to me.
In any case, a surprising pivot by Intellia for AAT lung disease and we
will have to see how this approach can thread the needle of achieving
substantial, but not exaggerated amounts of AAT….over the long-term…and with
little interpatient variability.
Intellia intends to submit a clinical trial application for NTLA-3001 by
year-end.