Back from recent CRISPR conferences in Ireland and Denmark, I wanted to share my current thinking about the present and foreseeable future of clinical genome editing.
CRISPR is a clinically mature platform
First of all, genome editing, and in particular CRISPR Therapeutics have arrived big time. Ignore for a moment the 90%+ declines in the share prices of Intellia, Verve, and Metagenomi, that all, with the exception of perhaps Beam Therapeutics, suggest that the industry will not survive. Focus instead on the transformational results last week by Verve Therapeutics which will shape the management of chronic metabolic and cardiovascular disease for decades to come, Beam Therapeutics last month announcing that it for the first time fixed a genetic disease (AATD) by reversing a point mutation, or the three ongoing phase 3 programs by Intellia Therapeutics which promise patients live unintruded by their disease.
So you say that this is only half the truth and many of them will develop cancer? With hundreds of subjects having been given CRISPR therapeutics, we still have yet to hear about such cases, including for the hematopoietic stem cell applications, probably the most stringent test in that regard given the history of retroviral gene therapy. This is supported by ample detailed genomic analyses demonstrating that many CRISPR modalities, in particular prime editing, often do not have any detectable off-targeting (!) and therefore are safer than going out in the sun or eating grilled meat. And don’t get me started with analysts and investors pretending that small molecule drugs, 'dirty drugs' by design, are much safer in that regard when many of them like methotrexate and hydroxyurea are known to cause cancer which barely gets a mention or is routinely accepted as part of the risk-benefit of a drug. Similarly, the currently more widely embraced antisense and RNAi technologies exhibit considerably more off-targeting, which in theory could result in cancer development with chronic use, than the average CRISPR therapeutic candidate today.
Prime editing is the ultimate CRISPR manifestation
Prime editing is the foreseeable endgame for genome editing involving edits up to 50 nucleotides. This is because in addition to being able to knock out genes like (Cas9) nuclease or base editing, it allows the developer to change nucleotides at will and with incredible specificity, and for gain-of-function. It has therefore surprised me to see that prime editing is still such a niche part in these conferences and barely used in industry and even academia where IP is less of an issue. This can likely be explained with it requiring a good deal of mental gymnastics when devising a prime editing strategy and subsequent tinkering to optimize efficacy. Prime editing will likely also be part of strategies for achieving even larger edits, including whole gene replacements that are often desirable.
In some ways this is reminiscent of zinc fingers which make for poor screening and quick molecular biology tools and were quickly forgotten when CRISPR came along with the promise of simple sgRNA design. However, a panel at the Copenhagen CRISPR meeting by genome editing veterans made it clear that when optimized, zinc fingers can be every bit as powerful as CRISPR and currently have a leg up where delivery is going to be viral for some time to come.
This is particularly true for the CNS, the fourth major tissue/organ target for genome editing applications after the liver, hematopoietic stem cell, and immune cells. Not only is it impossible to fit in prime and base editors in single AAV vectors, expressing a CRISPR effector nuclease of bacterial origin for years may be tricky (but not unsolvable) in terms of immunogenicity. There is also some unease about having nucleases loiter around in cells for years after their job has been done. Self-targeting/inactivation strategies may here be called for. Zinc fingers by being small and mimicking human proteins by contrast are ready for clinical use, a fact, as the deal flow by Sangamo Therapeutics illustrates, is increasingly being recognized by the wider industry.
Genome editing versus non-permanent modalities
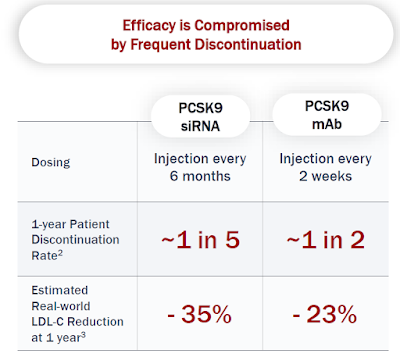
Another popular argument against CRISPR Therapeutics questions why one should take a ‘risk’ with permanently altering the genome when you have oligonucleotide therapeutics options that need to be administered as infrequently as every quarter, half annually, or even annually.
While there is some application overlap between genome editing and oligonucleotide therapeutics, especially when it comes to knocking down genes, a closer look shows that the overlap is limited. Where there is clear genetic evidence that a gene can be disabled for life without safety risks, then genome editing is the endgame, or at least capture a good portion of the market, and transient modalities may serve to de-risk the targets first.
When it comes to bringing pathways back into finely tuned balance and/or the disease is not chronic and likely requires only temporary treatment (e.g. some cases of hypertension), transient options are preferable. Still in other cases such as prion or Huntington’s disease where it is not clear whether it is the overall level or distribution of gene knockdown that is more important for efficacy, the preferred modality has yet to emerge from clinical studies.
Part of the Wall Street fashion show
Wall Street is a fashion show when it comes to biotech platforms. A little less than 10 years ago, it predicted that CRISPR would kill off the RNAi Therapeutics industry. Today, Alnylam sports a $30B market cap, about 4x that of the entire CRISPR industry combined (much of it in cash). Of course, investing in technologies ahead of their time is fatal. The electric vehicle industry is just one example. As I have laid out above, genome editing, however, is now and here to stay. Having the potential to save the healthcare system money (e.g. $2M for a one-and-done HAE/ATTR treatment as opposed to $500k annually) or to lead to drastically improved outcomes (as can be predicted from chronic LDLc lowering) got to be embraced by patients, their caregivers, docs, regulators, and insurers alike.
It's been a rough couple of years investing in development-stage, cutting-edge biotechs and current politics does not seem helpful, but if this is when it is darkest, then generational wealth awaits the survivors with the guts to hold on during a volatile comeback. One of my two conviction bets that I look up to in this regard is therefore CRISPR therapeutic biotech Verve Therapeutics. Uniqure, which works in the similarly unloved gene therapy arena and has a promising DNA-directed RNAi candidate for Huntington’s disease, is the other in case you were wondering.